-
- au
Ici vous trouverez le papier de Claire Verrier
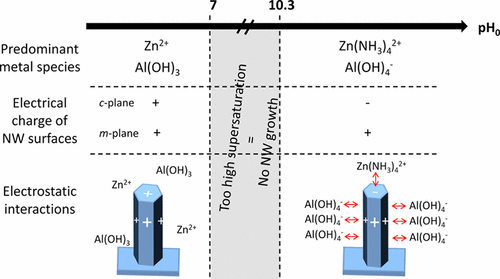
"The elucidation of the fundamental processes in aqueous solution during the chemical bath deposition of ZnO nanowires (NWs) using zinc nitrate and hexamethylenetetramine is of great significance: however, their extrinsic doping by foreign elements for monitoring their optical and electrical properties is still challenging. By combining thermodynamic simulations yielding theoretical solubility plots and speciation diagrams with in situ pH measurements and structural, chemical, and optical analyses, we report an in-depth understanding of the pH effects on the formation and aluminum doping mechanisms of ZnO NWs. By the addition of aluminum nitrate with a given relative concentration for the doping and of ammonia over a broad range of concentrations, the pH is shown to strongly influence the shape, diameter, length, and doping magnitude of ZnO NWs. Tuning the dimensions of ZnO NWs by inhibition of their radial growth only proceeds over a specific pH range, where negatively charged Al(OH)4– complexes are predominantly formed and act as capping agents by electrostatically interacting with the positively charged m-plane sidewalls. These complexes further favor the aluminum incorporation and doping of ZnO NWs, which only operate over the same pH range following thermal annealing above 200 °C. These findings reporting a full chemical synthesis diagram reveal the significance of carefully selecting and following the pH to control the morphology of ZnO NWs as well as to achieve their thermally activated extrinsic doping, as required for many nanoscale engineering devices."


